Topics and Sub Topics in Class 9 Science Chapter 4 Structure of Atom:
- Structure of Atom
- Charged Particles in Matter
- The Structure of an Atom
- How are Electrons Distributed in Different Orbits (Shells)?
- Valency
- Atomic Number and Mass Number
Q 1- Compare the properties of electrons, protons and neutrons.
Ans-
Particle | Nature of charge | Mass | Location |
Electron | Negative (–1) or (–1.6 x 10–19 C) | 9 x 10–31 kg | Extra nuclear part distributed in different shell or orbits. |
Proton | Positive (+1) or (+1.6 x 10–19 C) | 1.672 x 10–27 kg (1 µ) (approx. 2000 times that of the electron) | Nucleus |
Neutron | No charge | 1.672 x 10–27 kg (1 µ) (mass is nearly equal; to the mass of a proton) | Nucleus |
Q No. 2- What are the limitations of J.J. Thomson’s model of the atom?
Ans: According to J.J. Thomson’s model of an atom, it consists of a positively charged sphere with neutrons embedded in it. However, it was later found that the positively charged particles reside at the center of the atom called the nucleus, and the electrons revolve around the nucleus.
Moreover, Thomson attributed that the mass of an atom due to electrons and protons are evenly spread throughout the atom. This is not in agreement with the observations of Rutherford who concluded that mass is concentrated in a very small space in the center of the atom later called nucleus.
Q No. 3- What are the limitations of Rutherford’s model of atom?
Ans: According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed circular orbits. We know, any particle revolving in a circular orbit would experience acceleration. Due to this acceleration, the electrons revolving in circular orbits will lose energy in the form of radiation and finally fall into the nucleus. In such a case, atom would be highly unstable which is not true.
Q No. 4- Describe Bohr’s model of atom.
Ans: Bohr’s model of Atom
According to Bohr’s theory:

(1) The atom consists of a small positively charged nucleus at its center.
(2) The whole mass of the atom is concentrated at the nucleus and the volume of the nucleus is much smaller than the volume of the atom.
(3) All the protons and neutrons of the atom are contained in the nucleus.
(4) Only certain orbits known as discrete orbits of electrons are allowed inside the atom.
(5) While revolving in these discrete orbits electrons do not radiate energy. These orbits or cells are represented by the letters K, L, M, N etc. or the numbers, n = 1, 2, 3, 4, . . as shown in the above figure.
Q No.5- Compare all the proposed models of an atom given in this chapter.
Ans:
Thomson’s Model | Rutherford’s Model | Bohr’s Model |
1. An atom consists of a positively charged sphere and the electrons are embedded in it. 2. The negative and positive charges are equal in magnitude. As a result the atom is electrically neutral. | 1. An atom consists of a positively charged center in the atom called the nucleus. The mass of the atom is contributed mainly by the nucleus. 2. The size of the nucleus is very small as compared to the size of the atom. 3. The electrons revolve around the nucleus in well-defined orbits. | 1. Bohr agreed with almost all points as said by Rutherford except regarding the revolution of electrons for which he added that there are only certain orbits known as discrete orbits inside the atom in which electrons revolve around the nucleus. 2. While revolving in its discrete orbits the electrons do not radiate energy. |
Q No.6- Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Ans: Following rules are followed to fill electrons in different energy levels.
- If n gives the number of orbit or energy level, then 2n2 gives the maximum number of electrons possible in a given orbit or energy level. Thus,
First orbit or K-shell will have 2 electrons,
Second orbit or L-shell will have 8 electrons,
Third orbit or M-shell will have 18 electrons.
- If it is the outermost orbit, then it should have not more than 8 electrons.
3. There should be step-wise filling of electrons in different orbits, i.e., electrons are not accompanied in a given orbit if the earlier orbits or shells are incompletely filled.
Q No.7- Define valency by taking examples of silicon and oxygen.
Ans: The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element.
If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons and so, it has valency equal to four.
On the other hand, if the number of the number of valence electrons of the atom of an element is greater than 4, then the valency of that element is obtained by subtracting the number of valence electrons from 8. For example, the atom of oxygen has 6 valence electrons. and so, the valency of oxygen is (8 – 6) 2.
Q No.8- Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
Ans:
Atomic Number is defined as the number of protons present in the nucleus of an atom. For example, there are 6 protons in carbon, so the atomic number of carbon is 6. All atoms are characterized by their atomic numbers.
Mass Number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. For example, there are 6 protons and 6 neutrons in the nucleus of carbon, so its mass number is 12.
Isotopes are atoms of the same element thus having same atomic number but different mass number. For example, chlorine has two isotopes with atomic number 17 but mass numbers 35 and 37 represented by –
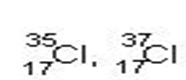
Two Uses of Isotopes
(a) Isotope of cobalt (60Co) is used in the treatment of cancer.
(b) Isotope of uranium (235U) is used as a fuel in nuclear reactors.
Isobars are such atoms which have same mass number but different atomic numbers. Thus, isobars are different elements. For example, Ne has atomic number 10 and sodium has atomic number 11 but both of them have mass numbers as 22 represented by –

Q No.9- Na+ has completely filled K and L shells. Explain.
Ans: The atomic number of sodium is 11. So, neutral sodium atom has 11 electrons and its electronic configuration is 2, 8, 1. But Na+ has 10 electrons. Out of 10, K-shell contains 2 and L-shell 8 electrons respectively. Thus, Na+ has completely filled K and L shells.
Q No.10- If bromine atom is available in the form of, say, two isotopes 7935Br (49.7%) and 8135Br (50.3%), calculate the average atomic mass of bromine atom.
Ans: Isotope of bromine with atomic mass 79 u = 49.7%
Contribution of 79Br to atomic mass of bromine

Q No.11- The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Ans:

Q No.12- If Z=3, what would be the valency of the element? Also name the element.
Ans: If Z=3, i.e., atomic number is 3. The element is lithium and has distribution of electrons as 2, 1.
And so, lithium has a valency of 1.
Q No.13- Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Ans:
Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
Since X and Y both have atomic numbers as 6 but mass numbers are different, therefore, these are isotopes.
Q No.14- For the following statements, write T for True and F for False.
(a) JJ Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Ans: (a) F (b) F (c) T (d) T
Put tick (√) against correct choice and cross (X) against wrong choice in the following questions:
Q No.15- Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus (b) Electron (c) Proton (d) Neutron.
Ans: (a) √ (b) X (c) X (d) X
Q No.16- Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers.
Ans: (a) X (b) X (c) √ (d) X
Q No.17- Number of valence electrons in Cl– ion is:
(a) 16 (b) 8 (c) 17 (d) 18
Ans: (a) X (b) √ (c) X (d) X
Q No.-18- Which one of the following is a correct electronic configuration of sodium?
(a) 2,8 (b) 8,2,1 (c) 2,1,8 (d) 2,8,1
Ans: (d)
NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame
NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame Topics and Sub Topics in Class 8 Science Chapter 6 Materials combustion and flame: Section Name Topic Name 6 Materials combustion and flame 6.1 What is Combustion? 6.2 How Do We Control Fire? 6.3 Types of Combustion 6.4 Flame
NCERT Solutions for Class 8 Science Chapter 5
NCERT Solutions for Class 8 Science Chapter 5 Materials Coal and Petroleum Topics and Sub Topics in Class 8 Science Chapter 5 Materials Coal and Petroleum: Section Name Topic Name 5 Materials Coal and Petroleum 5.1 Coal 5.2 Petroleum 5.3 Natural Gas 5.4 Some Natural Resources are Limited Coal and
NCERT Solutions for Class 8 Science Chapter 4 Materials Metals and Non Metals
NCERT Solutions for Class 8 Science Chapter 4 Materials Metals and Non Metals Topics and Sub Topics in Class 8 Science Chapter 4 Materials Metals and Non-Metals: Section Name Topic Name 4 Materials Metals and Non-Metals 4.1 Physical Properties of Metals and Non-metals 4.2 Chemical Properties of Metals and Non-metals